Thursday, April 3, 2014
Spore stain
Gram Stain
This is the most important stain in bacteriology and is so central to identification that it should be practised until the operator is fully competent. A number of different variations are found, and the laboratory should standardise on one method.
Saturday, March 29, 2014
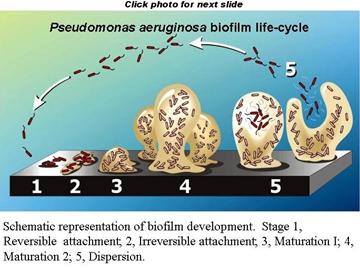
A General Model for Biofilm Development
Biofilm formation is
a developmental process in which bacteria undergo a regulated lifestyle switch
from a nomadic unicellular state to a sedentary multicellular state where
subsequent growth results in structured communities and cellular
differentiation. Results of prior work by many groups allow the construction of
a hypothetical developmental model for biofilm formation that can be
generalized for many different bacterial species. This model can be adjusted to
fit either of two general modes of unicellular lifestyle: nonmotile and motile.
Thursday, March 27, 2014
Transport of an Infectious Agent
Transmission involves
the transport of an infectious agent from the reservoir to the host. It is the
most important link in the chain of infection. Pathogens can be transmitted
from the reservoir to a susceptible host by various routes (Sobsey and Olson,
1983).
a. Person-to-Person
Transmission
The most common route
of transmission of infectious agents is from person to person. The best
examples of direct contact transmission are the sexually transmitted diseases
such as syphilis, gonorrhea, herpes, or acquired immunodeficiency syndrome
(AIDS).
Determination of Cell Biochemicals
Microbial biomass can
also be measured by determination of specific cell biochemical constituents
such as ATP, DNA, RNA, proteins, phospholipids, bacterial cell wall components,
or photosynthetic pigments (Sutton, 2002).
a.
ATP
Adenosine triphosphate
has often been used to determine live microbial biomass in environmental
samples, using a ratio of C/ATP
= 250 for aquatic
samples. However, the ATP content of cells varies with the growth rate and
metabolic state of microorganisms and nutrient limitation. A better measure is
the total
adenylate pool AT
(AT = ATP + ADP + AMP) because it does
not change greatly with changes in metabolic activities of the microorganisms.
The adenylate
energy charge (EC)
ratio provides information on growth potential of naturally occurring microbial
populations.
Tuesday, March 18, 2014
Fungi (Eukaryote)
Fungi are eukaryotic organisms that produce long filaments called hyphae,
which form a mass called mycellium. Chitin is a characteristic component of the
cell wall of hyphae. In most fungi, the hyphae are septate and contain
crosswalls that divide the filament into separate cells containing one nucleus
each. In some others,the hyphae are nonseptate and contain several nuclei. They
are called coenocytic hyphae.
Unusual Types of Bacteria (Part 3)
. Actinomycetes
Actinomycetes
are gram-positive filamentous bacteria characterized by mycelial growth (i.e.,
branching filaments), which is analogous to fungal growth. However, the diameter
of the filaments is similar in size to bacteria (approximately 1 mm).
Most actinomycetes are strict aerobes, but a few of them require anaerobic
conditions. Most of these microorganisms produce spores, and their taxonomy is
based on these reproductive structures (e.g., single spores in Micromonospora
or chains of spores in Streptomyces).
They are commonly found in water, wastewater treatment plants, and soils (with
preference for neutral and alkaline soils). Some of them (e.g., Streptomyces)
produce a
Saturday, March 15, 2014
Unusual Types of Bacteria (Part 2)
. Gliding
Bacteria
These
filamentous gram-negative bacteria move by gliding, a slow motion on a solid surface.
They resemble certain cyanobacteria except that they are colorless. Beggiatoa
and Thiothrix are gliding bacteria that
oxidize H2S to S0, which accumulates as sulfur granules inside the cells. Thiothrix
filaments are characterized by their ability to form rosettes. Myxobacteria are another group of
gliding microorganisms. They feed by lysing bacterial, fungal, or algal cells.
Vegetative cells aggregate to make “fruiting bodies,” which lead to the
formation of resting structures called myxospores. Under favorable conditions,
myxospores germinate into vegetative cells.
. Bdellovibrio
(B. bacteriovorus)
These
small (0.2–0.3 mm), flagellated (polar
flagellum) bacteria are predatory on gram-negative bacteria. After attaching to
the bacterial prey, Bdellovibrio penetrates the cells
and multiplies in the periplasmic space (space between the cell wall and the plasma
membrane). Because they lyse their prey, they are able to form plaques on a lawn
of the host bacterium. Some Bdellovibrio can
grow independently on complex organic media.
Wednesday, March 12, 2014
Unusual Types of Bacteria (Part 1)
. Sheathed
Bacteria
These
bacteria are filamentous microorganisms surrounded by a tubelike structure called
a sheath. The bacterial cells inside the sheath are gram-negative rods that become
flagellated (swarmer cells) when they leave the sheath. The swarmer cells produce
a new sheath at a relatively rapid rate. They are often found in polluted streams
and in wastewater treatment plants. This group includes three genera: Sphaerotilus, Leptothrix, and Crenothrix.
These bacteria have the ability to oxidize reduced
Friday, March 7, 2014
DNA Replication and Protein Synthesis
Replication:
The DNA moleculecan make an exact copy of itself. The two strands
separate and new complementary strands are formed. The double helix unwinds and
each of the DNA strands acts as a template for a new complementary strand.
Nucleotides move into the replication fork and
align themselves against the complementary bases on the template. The addition
of
Wednesday, March 5, 2014
Cytoplasmic Membrane (Plasma Membrane)
The cytoplasmic membrane is a 40–80 A ˚ -thick semipermeable membrane that contains a phospholipid bilayer with proteins embedded within the bilayer (fluid mosaic model) (Fig. 1.3). The phospholipid bilayer is made of hydrophobic fatty acids oriented towards the inside of the bilayer and hydrophilic glycerol moieties oriented towards the outside of the bilayer. Cations such as
Sunday, March 2, 2014
HACCP
There is growing acceptance throughout the EU and in many other
countries of the value of HACCP principles in ensuring the microbiological
safety of foods. The HACCP approach is a systematic way of analysing the
potential hazards of a food operation, identifying the points in the operation
where the hazards may occur, and where controls over those that are important
to consumer safety can be achieved. Most of the product-specific EC directives
as well as the Directive on the Hygiene of Foodstuffs (93/43/EEC), place
obligations on industry and food business operators to adopt HACCP principles
as the basis for their product safety management systems. The advantages of the
HACCP approach over a food safety control system based purely on
microbiological standards is now widely recognized. Thus, the Commission
proposes to consolidate and simplify existing EC food hygiene legislation. These are expected to be implemented by 2004. The proposed consolidation
adopts a unified approach to hygiene and extends the general hygiene rules and
HACCP principles to cover hygiene throughout the food chain, including primary
production, i.e. the ‘farm-to-fork’ approach to managing food safety.
Responsibility of food safety will be unambiguously placed onto food producers.
A fully documented HACCP plan will be required of all food producers, including
caterers, regardless of size.
This will include a specific monitoring programme, thereby
reinforcing the own-check principle of food producers. An absolute requirement
for full traceability of all foods and ingredients used in food production is
also introduced, such that all food producers must keep adequate records to
allow full traceability throughout the products’ allotted shelf-life.
Saturday, March 1, 2014
Measurement of Active Cells in Environmental Samples
Several
approaches have been considered for assessing microbial viability/activity
in environmental samples. Epifluorescence microscopy, in combination with the
use of oxido-reduction dyes, is used to determine the percent of active cells
in aquatic environments. The most popular oxido-reduction dyes are INT (2-(p-iodophenyl)-3-(p-nitrophenyl)-5-phenyl
tetrazolium chloride) and CTC (cyanoditolyl tetrazolium chloride) (Poschet al.,
1997; Pyle et al., 1995a). A good correlation was found between
Friday, February 28, 2014
Measurement of the Number of Viable Microbes on Solid Growth Media
This approach consists
of measuring the number of viable cells capable of forming colonies on a
suitable growth medium. Plate count is determined by using the pour plate
method (0.1–1 mL of microbial suspension is mixed with molten agar medium in a
petri dish), or the spread plate method (0.1 mL of bacterial suspension is
spread on the surface of an agar plate). The results of plate counts are
expressed as colony
forming units (CFU).
The number of CFU per plate should be between 30 and 300. Membrane filters can
also be used to determine microbial numbers in dilute samples. The sample is
filtered and the filter is placed directly on a suitable growth medium.
Thursday, February 27, 2014
Total Number of Microbial Cells
Total
number of cells (live and dead cells) can be measured by using special counting
chambers such as the Petroff–Hauser chamber for bacterial counts or the
Sedgewick–Rafter chamber for algal counts. The use of a phase-contrast
microscope is required when nonphotosynthetic microorganisms are under
consideration. Presently, the most popular method consists of retaining the cells
on a membrane filter treated to suppress autofluorescence (use of polycarbonate filters
treated with Irgalan Black) and staining the cells with fluorochromes such as
acridine orange (AO) or 40,6-diamidino-2-phenylindol
(DAPI). The microorganisms are subsequently counted using an epifluorescence
microscope (Kepner and Pratt, 1994).
An
advantage of DAPI is its stable fluorescence. A wide range of other
fluorochromes are available for many applications in environmental microbiology
studies. These include, among others, PicoGreen, SYBR-Green 1 and 2, Hoechst
33342, YOYO-1, and SYTO dyes (green, red, and blue) (Neu and Lawrence, 2002).
Scanning
electron microscopy (SEM) has also been considered for measuring total microbial
numbers. Electronic particle counters are also used for determining the total
number of microorganisms in a sample. These instruments do not differentiate,
however, between live and dead microorganisms, and very small cells may be
missed. Flow cytometers are fluorescence-activated cell sorters and include a
light source (argon laser or a mercury lamp) and a photodetector, which
measures fluorescence (use correct excitation wavelength) and scattering of the
cells. They sort and collect cells with predefined optical parameters. They are
often used in the biomedical and aquatic microbiology fields (Paul, 1993). They
have been used to sort algal cells and to distinguish between cyanobacteria
from other algae, based on phycoerythrin (orange) and chlorophyll (red)
fluorescence. They can help identify microorganisms when combined with
fluorescent antibodies.
Subscribe to:
Posts (Atom)